Back to: Fundamentals
what is electricity?
I want to start this fundamentals course by asking, what is electricity? I think that despite it being something that everyone is aware of, very few probably think about what it actually is and even fewer could form a definition! There are a number of ways of describing it, some of which I regularly see that are wrong! I think that electricity is so ubiquitous that most people never think about it. However, a good understanding of electricity will help so much with understanding many concepts in electronics.
what is an atom?
To answer this question, I think that a good starting point is to look at the atom. What is an atom? An atom is a small piece of matter that forms every element. Every atom is composed of different subatomic particles. When discussing electricity, we are primarily concerned with two of these particles – the proton and the electron. The proton is a positively charged particle, that is found in the nucleus of the atom, which is in the centre. The electron is a negatively charged particle that spins around the nucleus. Every element has a different number of electrons and protons. The number of protons determines which element is which. Hydrogen has 1 proton, helium has 2 protons and lithium has 3 protons.
hydrogen
Hydrogen is a good element to look at when looking to understand what an atom is. This is because it contains one electron and one proton, see figure 1. Protons and electrons are attracted to each other, which keeps them near to each other. Although electrons and protons have a charge, the overall charge of the atom is neutral because there is one positive and one negative – these cancel each other out.
Bohr model
There have been many models that have been devised to aid in understanding the atom. The one shown in figure 1 is the Bohr or planetary model. This is not the most current model but it serves well as a starting point for understanding the atom. It is commonly called the planetary model because it is similar to a planet orbiting the sun. The sun and planets are kept in orbit because of gravity, with electrons and protons the equivalent is known as an electrostatic force. This is a simplified model of our current understanding of an atom but this abstraction serves us well for this purpose. It makes it easy to see that the number of protons equals the number of electrons.
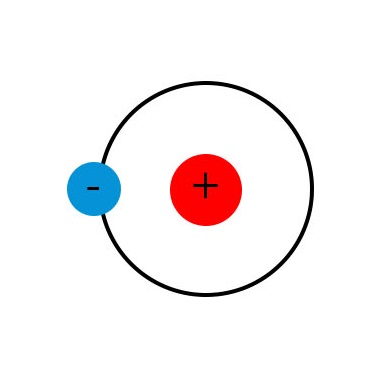
Now, an interesting thing about electrons is that they can leave their atom! If a hydrogen atom lost its electron it would now have a positive charge. Also, if that electron then joined another atom, that atom would now have a negative charge.
current electrcity
Now let’s look at a real-life circuit, where we can see electricity in action. Look at figure 2, where we have a power source (a battery), connected to a light bulb. I think that most people would correctly guess that the lightbulb will light up and that the circuit is working because of electricity! The electricity is coming from the battery, passing through the lightbulb and back to the battery. The battery is composed of chemicals and metals that are specifically chosen to create a positive and negative side. When there is a closed-circuit, such as in figure 2, charged particles move from the negative side of the battery, to the lightbulb and back to the positive side of the battery.
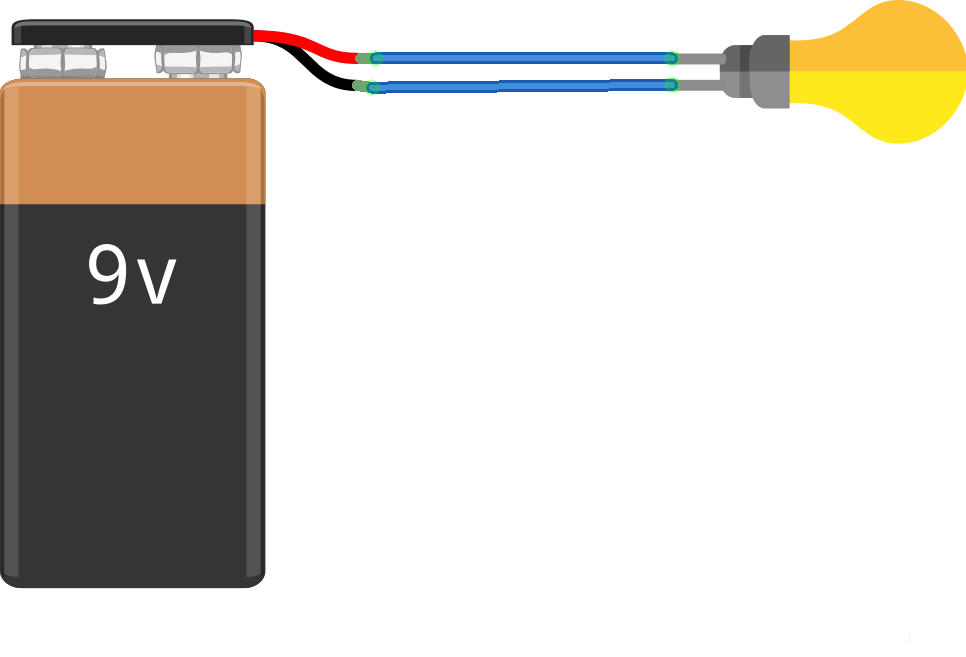
Therefore, we could say that electricity is the movement of charged particles! This is only partially correct, as there is more to it! The sort of electricity that we have just looked at is moving, so we call it current.
static electricity
There is another form of electricity, static. Most people have seen this in some form: lightning, rubbing your feet against a carpet and touching metal or making your hair stand up when you have taken a jumper off. To understand how this works, let’s look at a scenario.
If I wear a jumper made of wool and take a balloon made of rubber and rub the balloon against the jumper, there will be a transfer of charged particles. This is because the balloon and jumper are insulators, allowing electrons to transfer from the jumper to the balloon. This leaves the balloon with a negative charge. If you touch the balloon you may feel a shock as the electricity passes through you. Static charges build up on these items because they are good insulators of electricity.
triboelectric series
I was intentionally specific about the materials of the balloon and jumper because they work well for producing static electricity! There are plenty of other materials that could be used but some work better than others. There is actually a list, known as the triboelectric series, that ranks materials according to their tendency to lose and gain electrons.
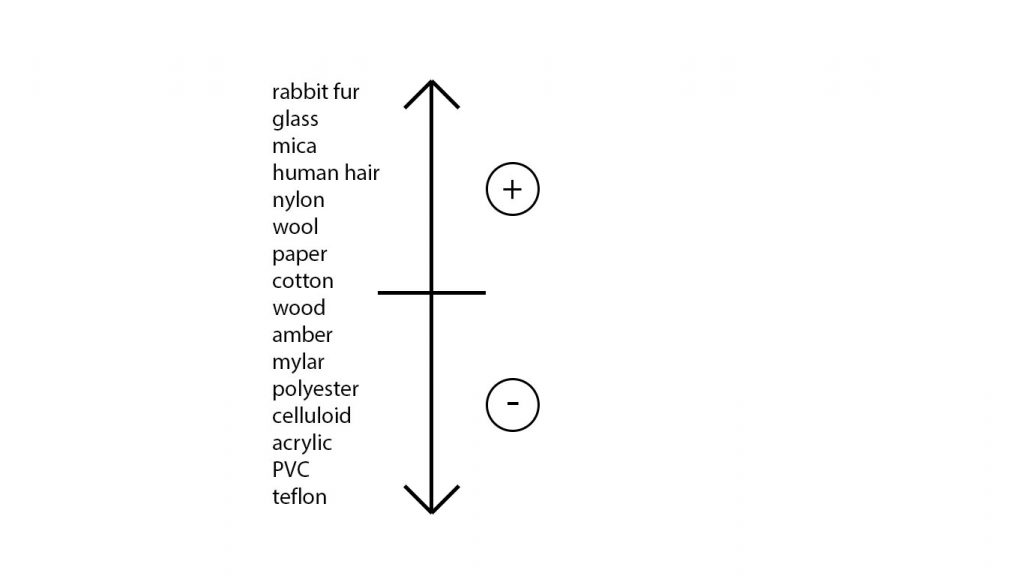
Figure 3 shows a list that I have compiled of some materials and where they sit on the scale. Items from the middle to the top acquire a positive charge when rubbed against an item from the middle to the bottom, which acquire a negative charge. Note that items don’t have to be rubbed but the action of rubbing increases the amount of contact and therefore helps to build up a charge!
After rubbing the balloon against the jumper, if you then hold it above small pieces of paper, the paper will ‘jump’ and stick to the balloon. If you look at the triboelectric scale, you will see that paper acquires a positive charge and therefore will be attracted to items that acquire a negative charge, such as the rubber of the balloon.
In conclusion, I would describe electricity as the presence or movement of charged particles. This statement then covers both static and current electricity.